Using Code ICD-10-CM for Cognitive Testing: Differentiating Between Mild and Major Cognitive Impairment
Published: 09/07/2025 | 12 min read
Written by: Emily Montemayor, Medical Coding Support Manager
Reviewed by: Mike Battista, Director of Science & Research
Cognitive impairment is a growing concern in aging populations, affecting millions of people in the U.S., and rising in prevalence as the population gets older. Early recognition and accurate diagnosis are crucial for guiding treatment, planning interventions, and improving patient outcomes. However, distinguishing between mild cognitive impairment (also known as MCI, or mild neurocognitive disorder) and major neurocognitive disorder (commonly known as dementia) can be challenging, as symptoms often present on a continuum and may overlap.
Cognitive testing plays a pivotal role in improving diagnostic accuracy by providing objective, quantifiable cognitive function data. Standardized assessments help clinicians track cognitive changes over time, support differential diagnoses, and enhance coding specificity for reimbursement purposes. This article explores how cognitive testing aids in differentiating between MCI and major neurocognitive disorders, ensuring patients receive the most appropriate care while optimizing clinical documentation and coding accuracy.
Understanding the Differences Between Mild and Major Cognitive Impairment
Mild cognitive impairment (MCI)
Mild cognitive impairment (MCI) is characterized by a noticeable decline in cognitive abilities, such as memory, attention, or problem-solving, that goes beyond what is expected for a person’s age and education level but does not significantly interfere with daily functioning.
Crucially, diagnosis requires more than just low performance on cognitive tests; there must also be a reported concern about cognitive decline, either from the individual, a knowledgeable informant, or the clinician—this is referred to as subjective decline (Sachs-Ericsson et al., 2021).
Cognitive assessments, like those offered through Creyos, help quantify subjective decline by integrating patient-reported outcomes with objective test performance. Individuals with MCI may report forgetfulness, word-finding difficulty, or trouble concentrating, yet typically maintain independence in daily activities.
While normal aging can involve subtle changes in cognitive function, such as occasional forgetfulness, MCI entails more pronounced impairments (Petersen et al., 2018). Key distinctions include:
- Frequency and severity: Memory lapses in MCI occur more frequently and are more severe than typical age-related forgetfulness.
- Objective evidence: Cognitive testing reveals measurable deficits in individuals with MCI, whereas normal aging does not typically produce significant testable impairments.
- Preserved daily functioning: Despite cognitive challenges, those with MCI maintain independence in daily activities, unlike individuals with dementia.
Several risk factors may increase the likelihood of developing MCI, including:
- Age: Advancing age is a significant risk factor for cognitive decline and mild cognitive impairment (MCI). Many studies highlight the increased prevalence of cognitive issues with advancing age (Luo et al., 2023).
- Cardiovascular conditions: Hypertension, diabetes, and high cholesterol have been shown to contribute to cognitive impairment and an increased risk of MCI. For example, a recent study of UK military veterans found that diabetes and hyperlipidemia had a significant association with higher MCI incidence, alongside other vascular risk factors (Miller et al., 2024).
- Lifestyle factors: Physical inactivity, poor diet, smoking, and social isolation also elevate risk. Managing these lifestyle factors may reduce the likelihood or progression of cognitive decline (Luo et al., 2023).
- Genetic Factors: Specific genetic variants, notably the APOE ε4 allele, have been associated with increased risk of Alzheimer's-related MCI, underscoring the connection between genetic predisposition and disease development (Luo et al., 2023).
Individuals with MCI have an increased likelihood of progressing to dementia. Roughly two out of 10 people aged 65 or older with MCI are estimated to develop dementia over a one-year period. However, some individuals remain stable and may even experience improvement in their symptoms with time and early intervention.
Major neurocognitive disorder
Major neurocognitive disorder, commonly known as dementia, involves a significant cognitive decline in one or more domains—like memory, language, executive function, or perceptual-motor skills—that interferes with independence in daily activities, according to the latest criteria.
The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5), outlines the following criteria for diagnosis:
- Evidence of substantial cognitive decline from a previous level of performance in one or more cognitive domains.
- Cognitive deficits interfere with independence in everyday activities.
- The deficits do not occur exclusively in the context of delirium and are not better explained by another mental disorder.
Dementia profoundly affects an individual's ability to perform everyday tasks. The effects of major neurocognitive disorder can result in:
- Loss of independence: Difficulty managing finances, medications, and personal care is common as cognitive abilities decline (Cipriani et al., 2020).
- Behavioral changes: Many individuals experience mood swings, agitation, or social withdrawal, which can further interfere with daily routines and relationships (Norton et al., 2001).
- Safety concerns: Safety risks increase significantly—wandering behaviors occur in over 60% of individuals with Alzheimer’s or dementia, leading to dangers like falling, getting lost, and even serious injury (Cipriani et al., 2020).
These challenges not only diminish the quality of life for individuals but also place significant emotional and physical burdens on caregivers.
The Common Causes of Dementia (DSM-5 Classification)
DSM-5 classifies dementia under major neurocognitive disorder and outlines several potential underlying causes:
- Alzheimer's Disease: The most prevalent cause, characterized by amyloid plaques and tau tangles in the brain.
- Vascular Dementia: Results from impaired blood flow to the brain, often related to strokes or vascular conditions.
- Lewy Body Dementia: Characterized by abnormal protein deposits (Lewy bodies) in brain cells.
- Frontotemporal Dementia: Involves degeneration of the frontal and temporal lobes of the brain.
- Other causes: Some other causes of dementia include Parkinson’s disease, Huntington’s disease, traumatic brain injury, multiple sclerosis, and neuroinfectious conditions like HIV.
Understanding the distinctions between MCI and dementia is crucial for accurate diagnosis, prognosis, and management. Early identification enables timely interventions, which may slow the progression and improve the quality of life.
The Role of Cognitive Testing in Diagnosis
Accurate diagnosis of cognitive impairments is essential for effective patient care. Cognitive testing serves as a cornerstone in measurement-based care, offering assistance in distinguishing between normal age-related changes, mild cognitive impairment, and major neurocognitive disorders. These assessments provide objective data that inform clinical decisions, guide treatment planning, and facilitate early intervention.
Types of Cognitive Tests
Cognitive assessments range from brief screening tools to comprehensive evaluations, each serving distinct purposes in the diagnostic process.
Screening Tools
- Mini-Mental State Examination (MMSE): A widely used 30-point questionnaire designed to assess cognitive functions such as memory, orientation, language, and basic arithmetic. While quick to administer, the MMSE has limited sensitivity for detecting subtle cognitive deficits—particularly in early-stage mild cognitive impairment (MCI)—and may not adequately assess executive function (Sternin et al., 2019).
- Montreal Cognitive Assessment (MoCA): Covers similar cognitive domains as the MMSE but incorporates tasks that assess executive function and abstract reasoning, such as clock drawing and trail-making. MoCA has been shown to be more sensitive than MMSE for identifying MCI, though factors such as education level, language proficiency, and repeat testing can influence accuracy (Gagnon et al., 2013).
- Creyos Dementia Screener: The Creyos Health platform offers a modern, digital alternative to traditional screening tools like the MoCA. Its two-task MCI screener is designed to efficiently assess cognitive domains commonly affected in early impairment, including attention, processing speed, and executive functioning. The screener can be used as a standalone tool or to complement results from tests like the MoCA, providing additional granularity when initial screening results are inconclusive (Lupton et al., 2021).
Comprehensive Cognitive Assessment Tools
For a more detailed evaluation, healthcare providers often refer patients for full neuropsychological testing, typically conducted by a neuropsychologist or neurologist. These evaluations are considered the gold standard for diagnosing cognitive impairment and provide in-depth analysis across multiple domains, but they can be time-consuming, resource-intensive, and difficult to access.
As a more efficient and accessible alternative, validated digital platforms like Creyos offer comprehensive online cognitive assessments that can detect even subtle signs of cognitive decline, including impairments that traditional screeners may miss. These tools evaluate a broad range of cognitive domains such as memory, attention, executive function, and processing speed.
By supporting early detection and continuous monitoring, Creyos assessments empower healthcare professionals to make timely, data-driven decisions that enhance both diagnosis and long-term care planning. Importantly, the platform also promotes health equity, ensuring that individuals from diverse backgrounds receive accurate, unbiased evaluations.
Interpreting Test Results
Accurate interpretation of cognitive testing data is critical for distinguishing between normal aging, mild cognitive impairment, and major neurocognitive disorder (dementia). Understanding the clinical significance of subtle score variations enables providers to deliver more precise diagnoses, improve documentation, and guide early interventions. Tools like those offered by Creyos can assist by providing granular data across multiple cognitive domains, enhancing the ability to track progression over time and tailor care accordingly.
Cognitive testing provides objective data that helps differentiate between normal aging, MCI, and dementia:
- Normal aging: This is often characterized by occasional forgetfulness or slower information processing that does not interfere with independent living. Cognitive test scores generally fall within the expected range when adjusted for age.
- Mild Cognitive Impairment (MCI): This involves measurable cognitive decline beyond normal aging in areas like memory, attention, and executive function while preserving the ability to perform basic daily tasks. This stage can represent a transitional phase, and accurate detection is essential for proactive preventative measures or care planning.
- Dementia: This represents a more significant and often progressive cognitive decline that disrupts daily function and autonomy. Comprehensive assessments, like those offered through Creyos, help evaluate the severity and profile of impairment, supporting differential diagnosis (i.e., Alzheimer’s versus vascular dementia) and guiding long-term care strategies.
Enhancing Clinical Decision-Making Through Cognitive Testing
Incorporating cognitive testing into clinical practice enhances decision-making by:
- Enabling early detection: Establishing an objective baseline and identifying cognitive decline at an early stage allows for timely interventions that may slow progression and improve patient outcomes.
- Supporting objective monitoring: Regular assessments provide objective data to track cognitive changes over time, informing treatment adjustments.
- Aiding with personalized care planning: Detailed cognitive profiles enable tailored care plans that address individual patient needs and support caregivers effectively.
Utilizing validated tools like Creyos assessments supports healthcare providers in delivering comprehensive cognitive care, from screening and diagnosis to personalized intervention strategies.
The Impact of Cognitive Testing on Patient Outcomes and Clinical Care
Precise cognitive diagnosis enables timely interventions that can slow disease progression and improve quality of life. Accurate and timely diagnosis means:
- Earlier intervention allows for lifestyle modifications, cognitive training, and treatment planning before a more significant decline.
- Tailored treatment plans, including medication adjustments, memory aids, and caregiver support, can be initiated sooner.
- Improved patient and caregiver education, ensuring that individuals and families understand the diagnosis, prognosis, and resources available for care planning.
Accurate and timely diagnosis, along with precise coding, timely diagnosis is crucial to enhancing clinical outcomes and care coordination by enabling personalized treatment and facilitating effective resource allocation (Robinson et al., 2015).
How Cognitive Testing Supports Longitudinal Care
Cognitive testing is not a one-time event but a tool for ongoing assessment over time.
- Monitoring disease progression: Regular reassessments help providers evaluate how quickly cognitive function is changing and whether treatments are effective.
- Guiding care transitions: Test results can inform decisions about increasing supervision, transitioning to assisted living, or involving home care services.
- Reducing healthcare costs: Early and ongoing detection has been shown to delay institutionalization and prevent avoidable hospitalizations by supporting proactive care strategies (Boustani et al., 2003).
Creyos offers automated testing intervals and tracking features that allow for objective, repeatable measurements of cognitive change over time.
Cognitive Testing Empowers Providers Through Precision
Cognitive testing is a powerful tool that enhances both clinical care and documentation accuracy. It enables providers to detect cognitive decline earlier, refine diagnoses, support medical necessity, and confidently code for the severity and subtype of impairment. In an era of value-based care, the ability to link objective test data with improved outcomes and reimbursement accuracy is more important than ever. Doing so not only improves patient care but also strengthens documentation, supports compliance, and aligns with risk-adjusted care models.
ICD-10-CM Coding for Cognitive Impairment
Accurate ICD-10-CM coding is essential for proper documentation, reimbursement, and care planning. The codes listed below are used to identify and classify cognitive impairment, including both dementia and mild cognitive impairment.
F01-F03 – Dementia Classifications
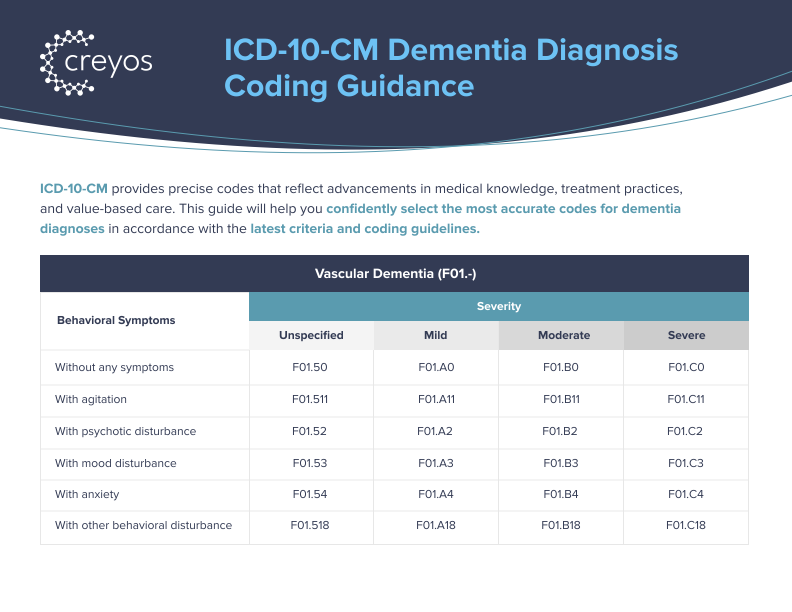
F01.xx – Vascular Dementia: Cognitive decline due to cerebrovascular disease or ischemic damage (I60-I69).
F02.xx – Dementia in Other Diseases Classified Elsewhere: Dementia resulting from conditions such as Huntington’s disease (G10) or Parkinson’s disease (G20.x).
F03.xx – Unspecified Dementia: Used when the specific etiology of dementia is unclear or has not yet been identified.
I69 – Sequela of Cerebrovascular Disease
Cognitive impairment following a cerebrovascular accident (CVA), such as a stroke, falls under this category. It’s important to specify the cause and degree of cognitive impairment to improve accuracy and guide treatment.
- F06.7x – Mild Neurocognitive Disorders due to Known Physiological Condition
Cognitive impairments caused by a specific medical condition, like Parkinson’s disease (G20.x) or Alzheimer’s disease (G30.x). This code is used when the impairment is directly linked to a known pathology.
Common Primary Etiologies
Each etiology has its own unique code and clinical implications. These include:
- G10 – Huntington's Disease: Neurodegenerative disorder causing motor dysfunction and cognitive decline.
- G20.x – Parkinson’s Disease: A movement disorder that can result in secondary cognitive decline.
- G30.0-G30.9 – Alzheimer’s Disease Classifications: Range of codes used to identify Alzheimer’s disease, based on stages and severity.
- G31.01 – Pick's Disease: A rare neurodegenerative disease leading to frontotemporal dementia.
- G31.09 – Other Frontotemporal Neurocognitive Disorder: Includes other types of frontotemporal degeneration affecting cognition.
- G31.83 – Neurocognitive Disorder with Lewy Bodies: Dementia caused by Lewy body pathology, with cognitive symptoms occurring before or within one year of motor signs. Distinct from Parkinson’s disease dementia, which is coded separately.
- G35 – Multiple Sclerosis: Autoimmune demyelinating disease of the CNS that may cause cognitive impairment, including slowed processing and memory decline.
- M32.x – Systemic Lupus Erythematosus (SLE): Autoimmune disease that can cause cognitive dysfunction due to neuropsychiatric involvement.
- S06.x – Traumatic Brain Injury: Post-injury cognitive deficits are captured with these codes. If traumatic brain injury leads to neurocognitive disorder, appropriate coding is essential for tracking recovery or long-term effects.
Other cognitive impairment reimbursement codes
- R41.81 – Age-Related Cognitive Decline: Age-associated changes in cognition that are not necessarily pathological but may be mistaken for early-stage dementia.
- G31.84 – Mild Cognitive Impairment, Unspecified: Used when a mild cognitive impairment diagnosis has been made, but no clear etiology has been identified.
Coding Considerations
To ensure accurate ICD-10-CM coding and optimal reimbursement, specificity in documentation is crucial. General terms like “cognitive impairment” or “memory loss” are insufficient and do not meet coding requirements. Providers should document:
- The specific type of cognitive impairment (e.g., Alzheimer’s disease, vascular dementia, or MCI).
- The severity of cognitive impairment (e.g., mild, moderate, or severe).
- Any contributing conditions (e.g., cerebrovascular disease, Parkinson’s disease, or traumatic brain injury).
- The patient’s functional status and impact on activities of daily living (ADLs) for impairment context.
Accurate documentation ensures the use of the correct ICD-10 code, which can affect patient care planning and risk-adjusted reimbursement models.
Linking Cognitive Testing Results to ICD-10-CM Codes
Cognitive testing results, such as those from digital cognitive tests (like those from Creyos) or traditional tests like the MoCA, provide critical evidence to support diagnosis. These test scores can be used to:
- Validate the clinical suspicion of cognitive decline
- Confirm or refine the dementia diagnosis (e.g., Alzheimer’s disease, vascular dementia)
- Track the progression or stability of cognitive function over time
- Differentiate between primary cognitive impairment (e.g., Alzheimer’s disease) and secondary cognitive impairment (e.g., post-stroke or trauma-related)
Using objective cognitive test data aligns with value-based care initiatives, enhancing the clinical picture and ensuring that the diagnosis is both accurate and substantiated by evidence.
Differentiating Primary vs. Secondary Cognitive Impairment
Accurate coding requires a clear distinction between primary and secondary cognitive impairments.
- Primary cognitive impairment is caused by neurodegenerative diseases such as Alzheimer’s disease (G30.x) or frontotemporal dementia (G31.x).
- Secondary cognitive impairment occurs when cognitive decline results from other medical conditions, such as cerebrovascular disease (I69), traumatic brain injury (S06.x), or systemic lupus erythematosus (M32.x).
Clear documentation of the underlying cause of cognitive impairment ensures the correct ICD-10-CM code is assigned, influencing both treatment strategies and reimbursement.
Enhancing Coding Accuracy Through Cognitive Testing
Cognitive testing should be considered medically necessary when a patient presents with symptoms like memory loss, confusion, impaired judgment, difficulty with language, or changes in executive function. These symptoms may emerge during wellness visits, routine screenings, or after caregiver reports. Testing is also warranted for patients with a history of neurological conditions, stroke, depression, or those at higher risk due to chronic comorbidities.
To support reimbursement, documentation should include:
- A clear clinical rationale for the test (e.g., cognitive decline noted on evaluation, family concerns, abnormal MoCA score).
- Relevant signs and symptoms (e.g., forgetfulness, impaired ADLs, personality changes).
- Medical history and risk factors (e.g., diabetes, cardiovascular disease, previous TBI).
- The intended use of results in care planning or diagnosis refinement.
Creyos offers structured report outputs and automated scoring, which support standardized documentation and can be easily integrated into EHRs for coding and billing purposes.
Using Test Results to Improve Diagnostic Coding
Cognitive test results help clinicians move beyond non-specific diagnoses like “memory loss” or “unspecified cognitive disorder” and toward accurate ICD-10-CM coding of cognitive conditions.
- Identifying dementia subtypes: Testing can support clinical differentiation between Alzheimer’s disease (G30.xx), vascular dementia (F01.xx), Lewy body dementia (G31.83), and frontotemporal dementia (G83.09). Combining test data with clinical history, neuroimaging, and biomarkers strengthens diagnostic accuracy and specificity (American Psychiatric Association, 2013).
- Assigning severity: DSM-5 and ICD-10-CM require severity classification (mild, moderate, or severe) for major neurocognitive disorders. Standardized cognitive scores provide an objective basis for assigning this severity, which is critical for appropriate coding, clinical decision-making, and treatment planning (American Psychiatric Association, 2013).
- Supporting risk adjustment: Accurate diagnosis and severity capture are vital for risk-adjusted payment models. Conditions like Alzheimer’s disease and other types of dementia carry significant weight in HCC and V28 risk adjustment models, directly affecting care coordination efforts and reimbursement outcomes (Centers for Medicare & Medicaid Services, 2024).
 Written by Emily Montemayor, Medical Coding Support Manager at Creyos
Written by Emily Montemayor, Medical Coding Support Manager at Creyos
With over a decade of experience in healthcare, Emily has finely honed skills in revenue integrity and auditing. Her current credentials include CCS, CMBCS, COC, CPC, and CPMA. She's been a trainer and educator who's had the opportunity to support over 50 hospitals across the US and internationally. She's deeply committed to optimizing reimbursement and enhancing revenue integrity by ensuring compliance with regulatory standards and continuously improving coding and auditing practices.
 Reviewed by Mike Battista, Director of Science & Research at Creyos
Reviewed by Mike Battista, Director of Science & Research at Creyos
Mike Battista specializes in brain health, cognition, and neuropsychological testing. He received his PhD in personality and measurement psychology at Western University in 2010 and has been doing fun and useful stuff in the intersection between science and technology ever since.